Heart Muscle Biology & Congestive Heart Failure
CVRTI’s Research on Heart Failure & Heart Biology
Heart failure is a clinical condition that results from an inability of the heart to pump blood efficiently. Patients with Heart failure are often subdivided by those with hearts that do not contract well (which is Heart Failure with reduced Ejection Fraction or HFrEF) and those with hearts that do not relax well in between contraction (which is Heart Failure with preserved Ejection Fraction, or HFpEF). Combined, there are 6 to 8 million Americans with heart failure, which has a high incidence of sickness (morbidity) and death (mortality). Additionally, the economic consequences of heart failure are devastating. Heart Failure related hospitalizations are a primary driver of our national Medicare and healthcare budget.
At the CVRTI, Investigators are researching heart biology and the mechanistic basis of heart failure focusing on studies in muscle biology. Our central hypothesis is that the more we learn about how heart muscle cells fail, the greater the chance we will be able to develop novel diagnostics and therapeutics for this devastating disease. Our efforts are already having success. Our investigators have developed novel blood-based diagnostics of failing heart muscle and have developed protocols for rescuing failing muscle on mechanical devices that can avoid future heart transplants and develop new gene therapies for failing heart muscle. Our ambitious goal is to change how heart failure is diagnosed and treated for the millions of patients who suffer from this disease.
Publications & Research
Association of a Novel Diagnostic Biomarker, the Plasma Cardiac Bridging Integrator 1 Score, with Heart Failure with Preserved Ejection Fraction and Cardiovascular Hospitalization.
This study introduces a novel, first in class, biomarker of heart failure. For a subset of patients who have heart failure with preserved ejection fraction (HFpEF), the biomarker (CS) recognizes heart remodeling and can predict which patients will suffer from future clinical decompensation.https://jamanetwork.com/journals/jamacardiology/fullarticle/2711896
Nikolova AP, Hitzeman TC, Baum R, Caldaruse A, Agvanian S, Xie Y, Geft DR, Chang DH, Moriguchi JD, Hage A, Azarbal B, Czer LS, Kittleson MM, Patel JK, Wu AHB, Kobashigawa JA, Hamilton M, Hong Thttps://cvrti.utah.edu/the-hong-laboratory/T, Shaw RM. Association of a novel diagnostic biomarker, the plasma cardiac bridging integrator 1 score, with heart failure with preserved ejection fraction and cardiovascular hospitalization. JAMA Cardiology, 2018; 3:1206-1210.
Auxiliary Trafficking Subunit GJA1-20k Protects Connexin43 from Degradation and Limits Ventricular Arrhythmias.
This study introduces how a protein formed by internal translation of Connexin43 mRNA is essential for the electrical communication of each heartbeat. Without it, arrhythmias and sudden death occur. In a broader sense, the study explains how previously unknown proteins are generated and required for heart function, expanding our body’s repertoire of protein diversity. https://www.jci.org/articles/view/134682
Xiao S, Shimura D, Baum R, Hernandez DM, Agvanian S, Nagaoka Y, Katsumata M, Lampe PD, Kleber AG, Hong T, Shaw RM. Auxiliary Trafficking Subunit GJA1-20k Protects Connexin43 from Degradation and Limits Ventricular Arrhythmias. JCI, 2020; 130(9):4858-4870.
Cardiac BIN1 Folds T-tubule Membrane, Controlling Ion Flux and Limiting Arrhythmia.
This study introduces a new concept of how heart muscle is organized and identifies a new cardiac protein (cardiac BIN1 or cBIN1), which is responsible for the microarchitecture of heart muscle cells. cBIN1 is important as both a diagnostic for patients with heart failure, and therapeutic for patients with either heart failure with reduced ejection fraction (HFrEF) or heart failure with preserved ejection fraction (HFpEF). https://www.nature.com/articles/nm.3543
Hong T, Yang H, Zhang S, Cho HC, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marbán E, Jan LY, Shaw RM. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nature Medicine, 2014; 20:624-32.
Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning.
This study discovered mechanical unloading induced up-regulation of glycolytic metabolites without a subsequent increase in pyruvate oxidation through the tricarboxylic acid (TCA) cycle. This investigation also demonstrated the effects of mechanical unloading on myocardial energetics, mitochondrial biogenesis, structure, and function. https://www.sciencedirect.com/science/article/pii/S2452302X16301097
Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY, Drakos SG. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl Sci, 2016; 1(6):432-444.
Identification of Nodal Tissue in the Living Heart Using Rapid Scanning Fiber-Optics Confocal Microscopy and Extracellular Fluorophores.
This study demonstrates feasibility of identifying nodal tissue in living heart using extracellular fluorophores and fiber-optics confocal microscopy. Application of the approach in pediatric reconstructive heart surgery may reduce risks of injuring nodal tissues. https://www.ahajournals.org/doi/full/10.1161/circimaging.112.000121
Huang C, Kaza AK, Hitchcock RW, Sachse FB. Identification of nodal tissue in the living heart using rapid scanning fiber-optics confocal microscopy and extracellular fluorophores. Circulation: Cardiovascular Imaging, 2013; 6(5), 739-746.
In Mice Subjected to Chronic Stress, Exogenous cBIN1 Preserves Calcium-Handling Machinery and Cardiac Function.
This study introduces the role of cBIN1-microdomains in organizing intracellular distribution of SERCA2a, which is disrupted in chronically stressed hearts with diastolic dysfunction. By normalizing cBIN1-microdomain via gene therapy, intracellular distribution of dyads and SERCA2a is reorganized to improve cardiac inotropy and lusitropy of failing hearts. https://www.sciencedirect.com/science/article/pii/S2452302X20301212?via%3Dihub
Liu Y, Zhou K, Li J, Agvanian S, Caldaruse AM, Shaw S, Hitzeman TC, Shaw RM, Hong T. In Mice Subjected to Chronic Stress, Exogenous cBIN1 Preserves Calcium-Handling Machinery and Cardiac Function. JACC Basic Transl Sci, 2020; 5(6), 561-578.
Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads.
This study introduces the role of cBIN1-microdomains in organizing L-type calcium channel and ryanodine receptor couplons at dyads. The study also identifies the super dynamic feature of cBIN1-microdomains, which reorganize within 5 minutes of beta-adrenergic activation for efficient excitation-contraction coupling during an acute stress response. https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.115.018535
Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T. Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads. Circulation, 2016; 133(4), 388-97.
Myocardial Atrophy and Chronic Mechanical Unloading of the Failing Human Heart: Implications for Ventricular Assist Devices-Induced Cardiac Recovery.
This study was the first to produce molecular, metabolic, histological, ultrastructural/EM, microstructural and functional data disproving the widely held view that chronic mechanical unloading leads to disuse myocardial atrophy that inhibits myocardial recovery. https://www.sciencedirect.com/science/article/pii/S0735109714057751
Diakos NA, Selzman CH, Sachse FB, Stehlik J, Kfoury AG, Reid BB, Miller DV, Salama ME, Fang JC, Drakos SG. Myocardial atrophy and chronic mechanical unloading of the failing human heart: implications for ventricular assist devices-induced cardiac recovery. J Am Coll Cardiol, 2014; 64:1602-12.
Sheet-Like Remodeling of the Transverse Tubular System in Human Heart Failure Impairs Excitation-Contraction Coupling and Functional recovery by Mechanical Unloading.
This study revealed that the transverse tubular system (t-system) in end-stage human heart failure presents a characteristic novel phenotype consisting of sheet-like invaginations of the sarcolemma. Our results suggest that the remodeled t-system impairs excitation-contraction coupling and functional recovery during chronic left ventricular assist device (LVAD) unloading. An intact t-system at the time of LVAD implantation may constitute a precondition and predictor for functional cardiac recovery after mechanical unloading. https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.116.024470
Seidel T, Navankasattusas S, Ahmad A, Diakos NA, Xu WD, Tristani-Firouzi M, Bonios MJ, Taleb I, Li DY, Selzman CH, Drakos SG, Sachse FB. Sheet-like remodeling of the transverse tubular system in human heart failure impairs excitation-contraction coupling and functional recovery by mechanical unloading. Circulation, 2017; 25;135(17):1632 45.
Subcellular Structures and Function of Myocytes Impaired During Heart Failure are Restored by Cardiac Resynchronization Therapy.
This study demonstrates remodeling of the transverse tubular system (t-system) and the spatial association of ryanodine receptor (RyR) clusters in a canine model of dyssynchronous heart failure. After CRT, we found a remarkable and almost complete reverse remodeling of these structures despite persistent left ventricular dysfunction. Studies of whole-cell calcium transients showed that the structural remodeling and restoration were accompanied with remodeling and restoration of calcium signaling. https://www.ahajournals.org/doi/full/10.1161/circresaha.111.257428
Sachse FB, Torres NS, Savio-Galimberti E, Aiba T, Kass DA, Tomaselli GF, Bridge JH. Subcellular structures and function of myocytes impaired during heart failure are restored by cardiac resynchronization therapy. Circulation Research, 2012; 110(4), 588-597.
The ESCRT-III Pathway Facilitates Cardiomyocyte Release of cBIN1-Containing Microparticles.
This study introduces the mechanistic turnover of cardiomyocyte cBIN1-microdomains from normal and failing cardiomyocytes. cBIN1 interacts with an ESCRT-III member CHMP4B to release cBIN1-microparticles from cardiomyocyte membrane microdomains into circulation. The released cBIN1-microparticles carry cardiac specific signals serving as a marker of biochemical health of cardiomyocytes. https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.2002354
Xu B, Fu Y, Liu Y, Agvanian S, Wirka RC, Baum R, Zhou K, Shaw RM, Hong T. The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol, 2017; 15(8), e2002354.
The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure.
Based on the previous study in JACC Basic Translational Science by Badolia et. al, we hypothesized that the accumulated glycolytic intermediates are channeled into accessory pathways of glucose metabolism that are cardioprotective and may induce myocardial recovery. In this follow-up study, we discovered that the recovering heart directs glycolytic metabolites into nonglycolytic glucose metabolism pathways such as pentose-phosphate and 1-carbon metabolism, which contributed to cardioprotection and myocardial repair by generating reduced NADP, enhancing biosynthesis and reducing oxidative stress. These discoveries provide insights into novel therapeutic targets for myocardial recovery. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.044452
Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Navankasattusas S, Wever-Pinzon O, Selzman CH, Chaudhuri D, Rutter J, Drakos SG. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure. Circulation, 2020; 142(3):259-274.


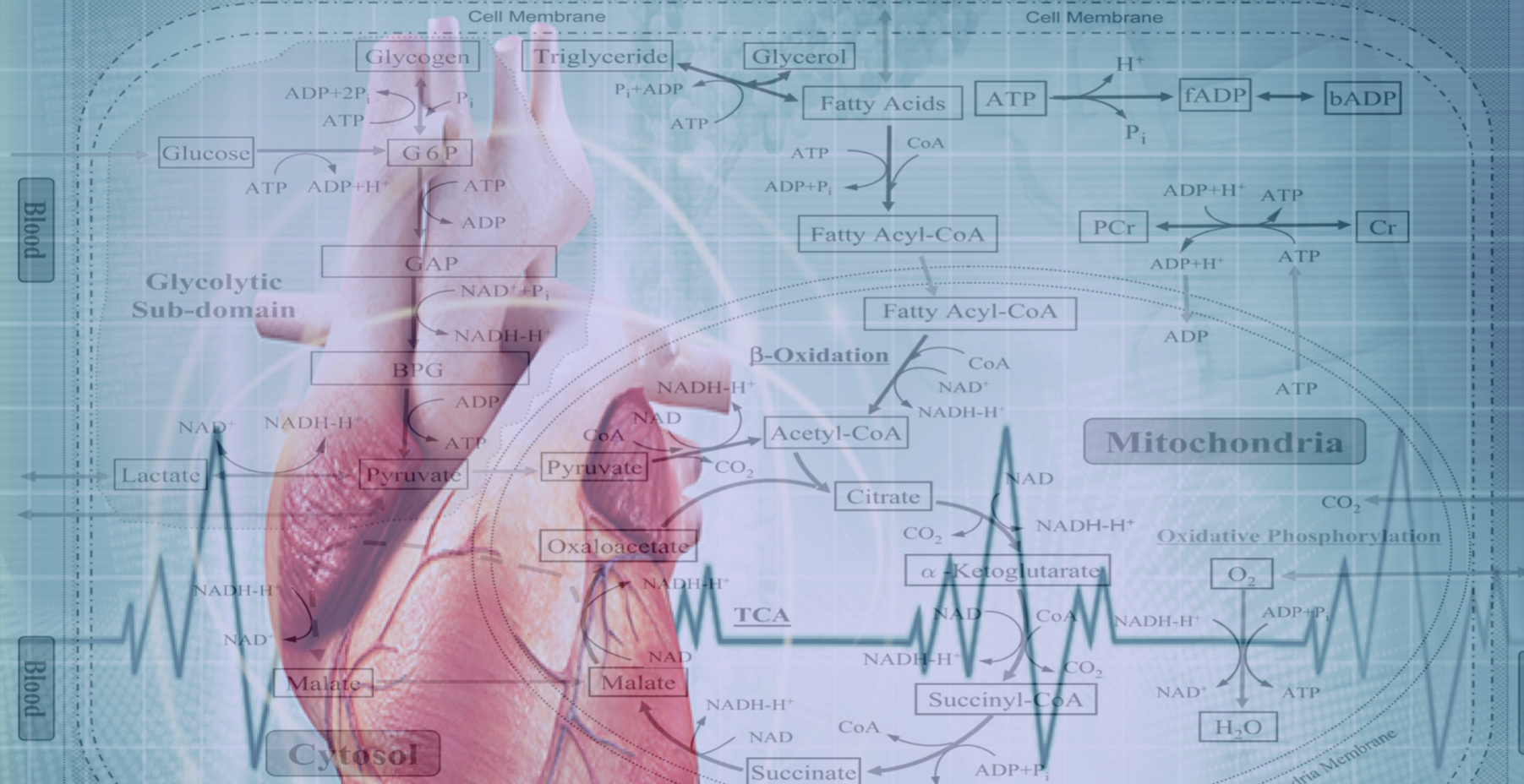 The Drakos Laboratory, headed by Stavros Drakos, MD, PhD, through an ongoing collaboration with the Sachse Laboratory, studies how cardiac ion channels are distributed and how channels clustered to specific subdomains of cardiac cell membrane affect excitation-contraction coupling in healthy, failing, and recovering heart muscle. Link to lab summary page on CVRTI
The Drakos Laboratory, headed by Stavros Drakos, MD, PhD, through an ongoing collaboration with the Sachse Laboratory, studies how cardiac ion channels are distributed and how channels clustered to specific subdomains of cardiac cell membrane affect excitation-contraction coupling in healthy, failing, and recovering heart muscle. Link to lab summary page on CVRTI 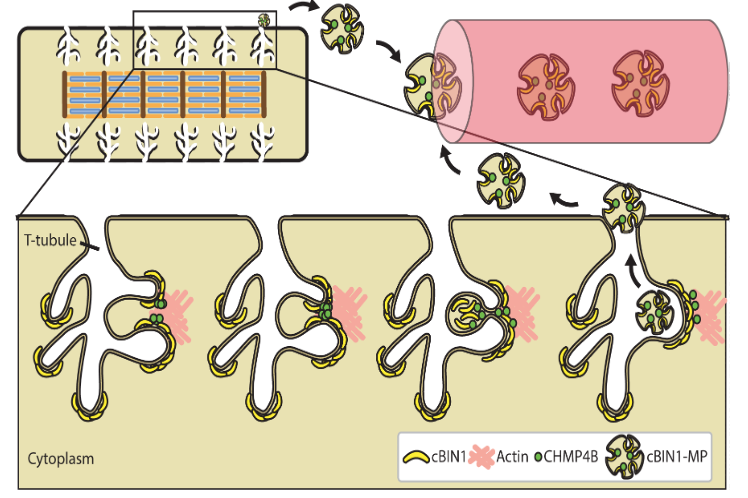 The Hong Laboratory, headed by TingTing Hong, MD, PhD, studies the regulation and remodeling of membrane microdomains of cardiomyocytes during heart failure progression. They study how cardiomyocyte surface microdomains are organized to concentrate ion channels and signaling proteins for proper function and regulation in normal and failing hearts. The research includes the mechanisms of scaffolding protein and cytoskeleton-based maintenance of membrane structures and subdomains important in calcium signaling, turnover mechanisms of microdomains, and the mechanisms of heart failure progression. The Hong lab’s goal is to identify, at the bench, new molecular and cellular targets that can be translated to develop new therapeutic tools for clinical management of heart failure.Link to lab summary page on CVRTI
The Hong Laboratory, headed by TingTing Hong, MD, PhD, studies the regulation and remodeling of membrane microdomains of cardiomyocytes during heart failure progression. They study how cardiomyocyte surface microdomains are organized to concentrate ion channels and signaling proteins for proper function and regulation in normal and failing hearts. The research includes the mechanisms of scaffolding protein and cytoskeleton-based maintenance of membrane structures and subdomains important in calcium signaling, turnover mechanisms of microdomains, and the mechanisms of heart failure progression. The Hong lab’s goal is to identify, at the bench, new molecular and cellular targets that can be translated to develop new therapeutic tools for clinical management of heart failure.Link to lab summary page on CVRTI 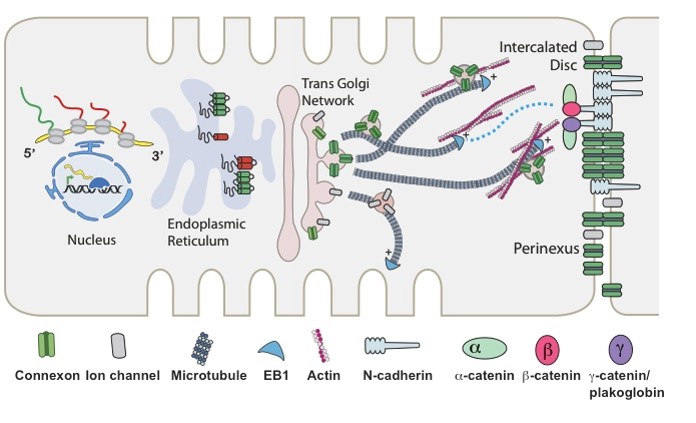 The Shaw Laboratory, headed by Robin Shaw, MD, PhD, study how cardiac ion channels are formed and how channels are targeted to specific subdomains of cardiac cell membrane in both healthy and failing heart muscle. Link to lab summary page on CVRTI
The Shaw Laboratory, headed by Robin Shaw, MD, PhD, study how cardiac ion channels are formed and how channels are targeted to specific subdomains of cardiac cell membrane in both healthy and failing heart muscle. Link to lab summary page on CVRTI 
