
The Hong Laboratory focuses on understanding the regulation and remodeling of membrane microdomains of cardiomyocytes during heart failure progression. We study how cardiomyocyte surface microdomains are organized to concentrate ion channels and signaling proteins for proper function and regulation in normal and failing hearts. The research interested includes the mechanisms of scaffolding protein and cytoskeleton-based maintenance of membrane structures and subdomains important in calcium signaling, turnover mechanisms of microdomains, and the mechanisms of heart failure progression. The goal is to identify, at the bench, new molecular and cellular targets that can be translated to develop new therapeutic tools for clinical management of heart failure.
Featured Publications
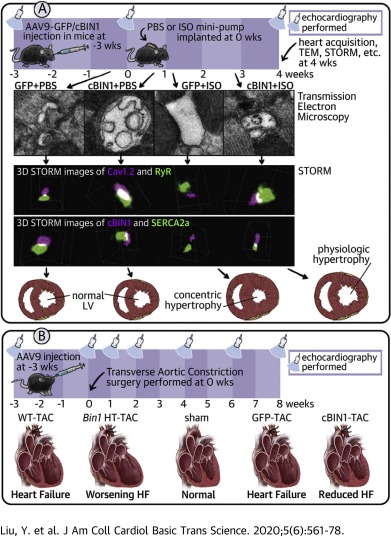 | In Mice Subjected to Chronic Stress, Exogenous cBIN1 Preserves Calcium-Handling Machinery and Cardiac Function https://www.sciencedirect.com/science/article/pii/S2452302X20301212?via%3Dihub | This study introduces the role of cBIN1-microdomains in organizing intracellular distribution of SERCA2a, which is disrupted in chronically stressed hearts with diastolic dysfunction. By normalizing cBIN1-microdomain via gene therapy, intracellular distribution of dyads and SERCA2a is reorganized to improve cardiac inotropy and lusitropy of failing hearts. | Liu Y, Zhou K, Li J, Agvanian S, Caldaruse AM, Shaw S, Hitzeman TC, Shaw RM, Hong T (2020). In Mice Subjected to Chronic Stress, Exogenous cBIN1 Preserves Calcium-Handling Machinery and Cardiac Function. JACC Basic Transl Sci, 5(6), 561-578. |
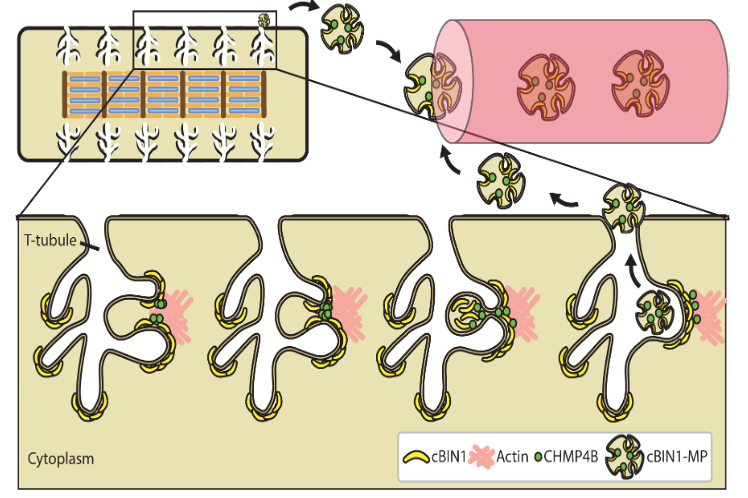
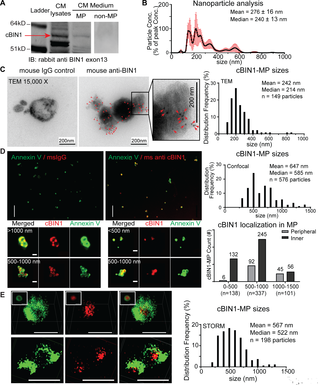 | The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.2002354 | This study introduces the mechanistic turnover of cardiomyocyte cBIN1-microdomains from normal and failing cardiomyocytes. cBIN1 interacts with an ESCRT-III member CHMP4B to release cBIN1-microparticles from cardiomyocyte membrane microdomains into circulation. The released cBIN1-microparticles carry cardiac specific signals serving as a marker of biochemical health of cardiomyocytes. | Xu B, Fu Y, Liu Y, Agvanian S, Wirka RC, Baum R, Zhou K, Shaw RM, Hong T (2017). The ESCRT-III pathway facilitates cardiomyocyte release of cBIN1-containing microparticles. PLoS Biol, 15(8), e2002354. |
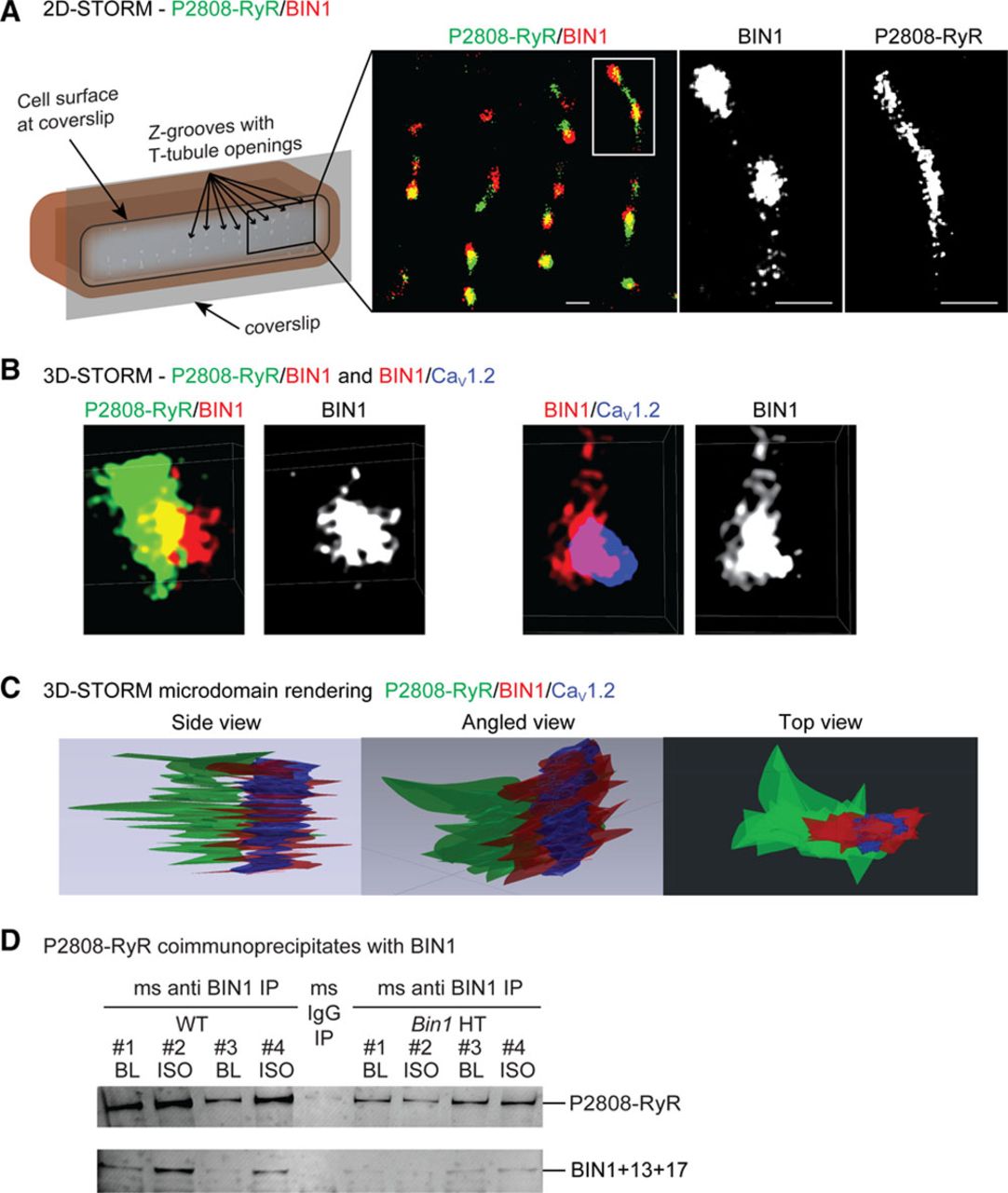 | Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.115.018535 | This study introduces the role of cBIN1-microdomains in organizing L-type calcium channel and ryanodine receptor couplons at dyads. The study also identifies the super dynamic feature of cBIN1-microdomains, which reorganize within 5 minutes of b-adrenergic activation for efficient excitation-contraction coupling during an acute stress response. | Fu Y, Shaw SA, Naami R, Vuong CL, Basheer WA, Guo X, Hong T (2016). Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads. Circulation, 133(4), 388-97. |










