Heart Failure Research and Cardiac Metabolism
Metabolism, for the heart, refers to the many chemical reactions inside heart muscle cells that require energy. Studies of heart metabolism usually involve the powerhouses of heart cells and powerhouses of every cell, known as mitochondria. Failing hearts have altered metabolism, which negatively affects heart contraction and relaxation and can be a primary cause of heart arrhythmias. At the CVRTI, a growing number of Investigators are performing pioneering studies in heart metabolism. If we can preserve a heart’s ability to produce energy and reduce the number of toxic waste products it generates, we can improve both heart function and prevent arrhythmias.
Publications
Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning.
This study discovered mechanical unloading induced up-regulation of glycolytic metabolites without a subsequent increase in pyruvate oxidation through the tricarboxylic acid (TCA) cycle. This investigation also demonstrated the effects of mechanical unloading on myocardial energetics, mitochondrial biogenesis, structure, and function. https://doi.org/10.1016/j.jacbts.2016.06.009
Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY,Drakos SG. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl Sci. 2016 Oct;1(6):432-444.
Histone Methyltransferase Smyd1 Regulates Mitochondrial Energetics in the Heart.
This study identifies the histone methyltransferase Smyd1 as a novel regulator of mitochondrial energetics in the heart. Integrated metabolomics and proteomics revealed that loss of Smyd1 in the adult mouse heart leads to global downregulation of mitochondrial proteins involved in oxidative phosphorylation. ChIP analysis and luciferase reporter assays further determined that Smyd1 positively regulates the expression of PGC-1, a master regulator of mitochondrial bioenergetics, through epigenetic modifications on the PGC-1 promoter. https://www.pnas.org/content/115/33/E7871.short
Warren JS, Tracy CM, Miller MR, Makaju A, Szulik MW, Oka SI, Yuzyuk TN, Cox JE, Kumar A, Lozier BK, Wang L, Llana JG, Sabry AD, Cawley KM, Barton DW, Han YH, Boudina S, Fiehn O, Tucker HO, Zaitsev AV, Franklin S. Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. Proc Natl Acad Sci U S A, 2018; 115(33):E7871-E7880.
Insulin receptor substrates differentially exacerbate insulin-mediated left ventricular remodeling
The failing heart exhibited divergent effect of IRS1/Akt1 axis, independent of IRS2 and Akt2 signaling in modulation of LV remodeling and contractile dysfunction in heart failure. The failing human heart was associated with hyperactivation of IRS1 and Akt1, but not IRS2 and Akt2. This study identified IRS1 as a potential regulator of cardioprotective protein kinase G-mediated signaling. https://doi.org/10.1172/jci.insight.134920.
Riehle C, Weatherford ET, Wende AR, Jaishy BP, Seei AW, McCarty NS, Rech M, Shi Q, Reddy GR, Kutschke WJ, Oliveira K, Pires KM, Anderson JC, Diakos NA, Weiss RM, White MF, Drakos SG, Xiang YK, Abel ED. Insulin receptor substrates differentially exacerbate insulin-mediated left ventricular remodeling JCI Insight. 2020 Mar 26;5(6):e134920.
Multiple Levels of PGC-1α Dysregulation in Heart Failure.
This review article summarizes how PGC-1α is regulated by a multitude of mechanisms in the healthy and failing hearts, operating at different regulatory levels, which include epigenetic regulation, the expression of variants, post-transcriptional inhibition, and post-translational modifications.https://www.frontiersin.org/articles/10.3389/fcvm.2020.00002/full
Oka SI, Sabry AD, Cawley KM, Warren JS. Multiple Levels of PGC-1α Dysregulation in Heart Failure. Front Cardiovasc Med, 2020; 7:2.
Perm1 Regulates Cardiac Energetics as a Downstream Target of the Histone Methyltransferase Smyd1.
This study identifies Perm1 (PPARGC-1 and ESRR-induced regulator, muscle specific 1) as a downstream target of the histone methyltransferase Smyd1. Perm1 is downregulated in the mouse and human failing hearts, while loss- and gain-of-function study reveals that Perm1 positively regulates mitochondrial energetics in cardiomyocytes. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0234913
Oka SI, Sabry AD, Horiuchi AK, Cawley KM, O’Very SA, Zaitsev MA, Shankar TS, Byun J, Mukai R, Xu X, Torres NS, Kumar A, Yazawa M, Ling J, Taleb I, Saijoh Y, Drakos SG, Sadoshima J, Warren JS. Perm1 regulates cardiac energetics as a downstream target of the histone methyltransferase Smyd1. PLoS One, 2020; 15(6):e0234913.
Stress Response Protein GJA1-20k Promotes Mitochondrial Biogenesis, Metabolic Quiescence, and Cardioprotection against Ischemia/Reperfusion Injury.
This study identifies that GJA1-20k mediates ischemia-preconditioning and the exogenous introduction of GJA1-20k could prevent anticipated ischemia related organ damage. https://insight.jci.org/articles/view/121900
Basheer WA, Fu Y, Shimura D, Xiao S, Agvanian S, Hernandez DM, Hitzeman TC, Hong TT, Shaw RM. Stress Response Protein GJA1-20k Promotes Mitochondrial Biogenesis, Metabolic Quiescence, and Cardioprotection against Ischemia/Reperfusion Injury. JCI Insight, 2018; 3(20).
The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure Circulation.
Based on the findings of the aforementioned study (#1) we hypothesized that the accumulated glycolytic intermediates are channeled into accessory pathways of glucose metabolism that are cardioprotective and may induce myocardial recover. Indeed, in this follow-up study we discovered that the recovering heart directs glycolytic metabolites into nonglycolytic glucose metabolism pathways such as pentose-phosphate and 1-carbon metabolism, which contributed to cardioprotection and myocardial repair by generating reduced NADP, enhancing biosynthesis and reducing oxidative stress. These discoveries provide insights into novel therapeutic targets for myocardial recovery. https://doi.org/10.1161/CIRCULATIONAHA.119.044452
Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Navankasattusas S, Wever-Pinzon O, Selzman CH, Chaudhuri D, Rutter J, Drakos SG. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure Circulation. 2020;142(3):259-274.
The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure.
Myocardial MPC expression coincides with LVAD-mediated recovery in chronic HF patients. Loss of the MPC in cultured cells and in murine hearts is sufficient to induce hypertrophy and HF. MPC overexpression attenuates drug-induced hypertrophy in a cell-autonomous manner. Inhibition of MCT4 can mitigate hypertrophy in cultured cardiomyocytes and in mice.https://www.sciencedirect.com/science/article/abs/pii/S1550413120306586
Cluntun AC*, Badolia R* (co-first authors), Lettlova S, Parnell KM, Shankar T, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Thodou A, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG. The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. Cell Metabolism, In Press

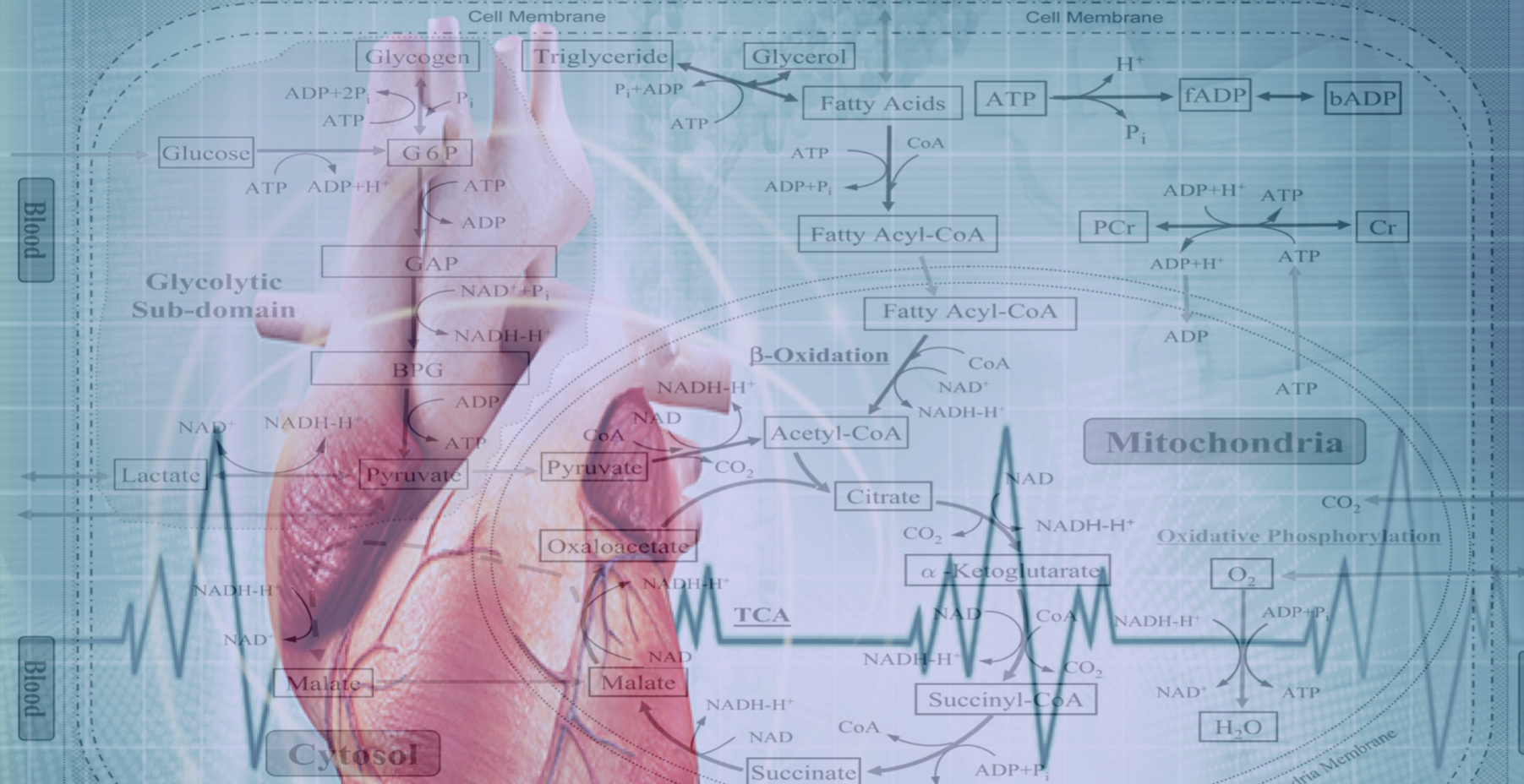 The Drakos Laboratory are focused on discovering metabolic adaptation that lead to pathological remodeling of the heart in chronic and acute heart failure. The lab is trying to understanding the mechanisms that can modify these metabolic pathways and restore cardiac structure and function and therefore discover new therapeutic targets for myocardial recovery.
The Drakos Laboratory are focused on discovering metabolic adaptation that lead to pathological remodeling of the heart in chronic and acute heart failure. The lab is trying to understanding the mechanisms that can modify these metabolic pathways and restore cardiac structure and function and therefore discover new therapeutic targets for myocardial recovery. 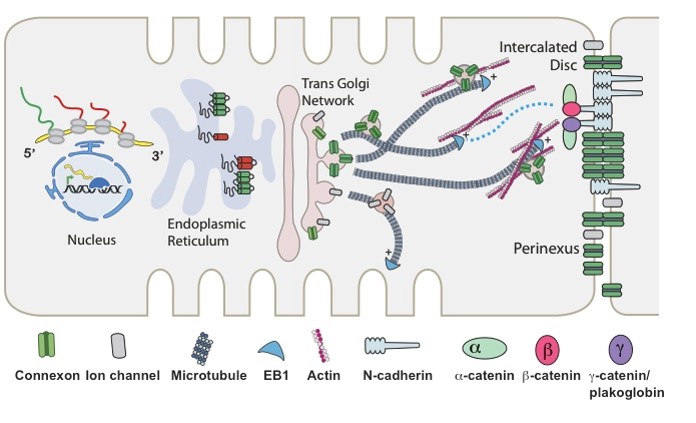 The Shaw Laboratory, headed by Robin Shaw, MD, PhD, studies how subunits of cardiac ion channels not just assist with ion channel trafficking, but affect mitochondrial dynamics and metabolism. Link to lab summary page on CVRTI
The Shaw Laboratory, headed by Robin Shaw, MD, PhD, studies how subunits of cardiac ion channels not just assist with ion channel trafficking, but affect mitochondrial dynamics and metabolism. Link to lab summary page on CVRTI 
