
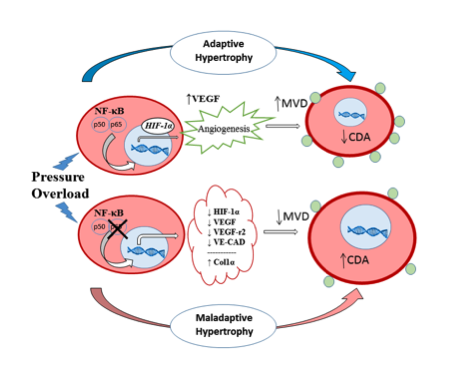
The Selzman Laboratory’s research utilizes models of pressure overload and subsequent relief to study remodeling and reverse remodeling in mice. We are looking at the transcriptional regulation of these pathologic events, and in particular, the role of nuclear factor kappa B.
Featured Publications
The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. | Myocardial MPC expression coincides with LVAD-mediated recovery in chronic HF patients. Loss of the MPC in cultured cells and in murine hearts is sufficient to induce hypertrophy and HF. MPC overexpression attenuates drug-induced hypertrophy in a cell-autonomous manner. Inhibition of MCT4 can mitigate hypertrophy in cultured cardiomyocyte and in mice. | Cluntun AC*, Badolia R* (co-first authors), Lettlova S, Parnell KM, Shankar T, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Thodou A, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG. The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. Cell Metabolism, In Press | |
The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure Circulation. | Based on the findings of the aforementioned study (#1) we hypothesized that the accumulated glycolytic intermediates are channeled into accessory pathways of glucose metabolism that are cardioprotective and may induce myocardial recover. | Badolia R, Ramadurai DKA, Abel ED, Ferrin P, Taleb I, Shankar TS, Navankasattusas S, Wever-Pinzon O, Selzman CH, Chaudhuri D, Rutter J, Drakos SG. The Role of Nonglycolytic Glucose Metabolism in Myocardial Recovery Upon Mechanical Unloading and Circulatory Support in Chronic Heart Failure Circulation. 2020;142(3):259-274. | |
Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. | This study discovered mechanical unloading induced up-regulation of glycolytic metabolites without a subsequent increase in pyruvate oxidation through the tricarboxylic acid (TCA) cycle. This investigation also demonstrated the effects of mechanical unloading on myocardial energetics, mitochondrial biogenesis, structure, and function. | Diakos NA, Navankasattusas S, Abel ED, Rutter J, McCreath L, Ferrin P, McKellar SH, Miller DV, Park SY, Richardson RS, Deberardinis R, Cox JE, Kfoury AG, Selzman CH, Stehlik J, Fang JC, Li DY, Drakos SG. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl Sci, 2016; 1(6):432-444. |
The Selzman Lab Research Team






