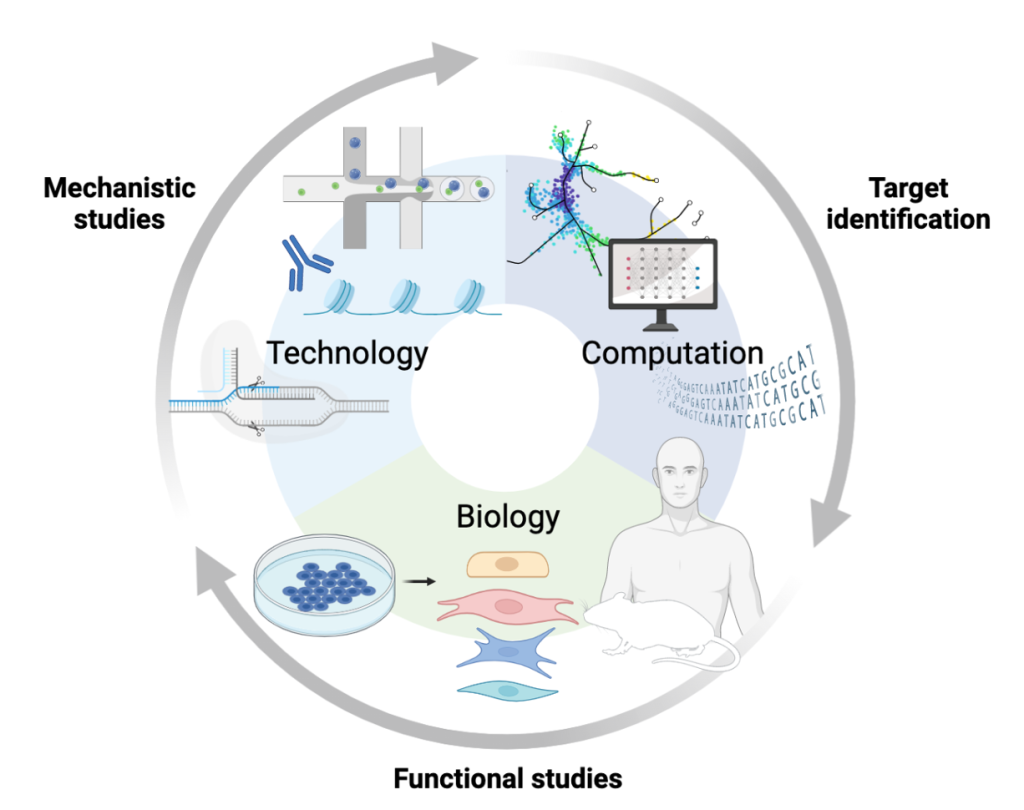
The Guo Laboratory’s research is focused on deciphering the pathogenicity of genetic variants in cardiovascular diseases. With the development of medical genetics and low-cost, rapid DNA sequencing technologies, a large number of genetic variants associated with cardiovascular diseases have been identified and released in population databases including ClinVar, ClinVar Miner, dbSNP, Exome sequencing project, 1000Genomes, and Human Gene Mutation Database. However, about 40% of total variants known as variants of uncertain significance, are still not clearly defined to be classified as pathogenic or benign. This gap in knowledge significantly obstructs potential widespread clinical application of the genetic information and causes anxiety and stress to genetic variant carriers. In addition, most of cardiovascular diseases are polygenic diseases that result from the additive inheritance of multiple genetic variants at different loci alongside interactions with environmental factors, culminating in an affected disease phenotype. Thus, our laboratory is thriving to understand the genetic mechanisms underlying cardiovascular diseases and develop precise prevention and treatment for genetic variants carriers. We utilize computational and biological systems such as stem cells, animal models, and human samples to address these questions.
Featured Publications
SGLT2i ameliorates endothelial dysfunction associated with the common ALDH2 alcohol flushing variant | We showed that ALDH2*2 contributes to impaired vascular function in humans by increasing oxidative stress and inflammation in the endothelium, which is a well-known contributor to coronary artery disease. We also reported that the diabetes drug empagliflozin (a sodium- glucose transporter 2 inhibitor) improved endothelial function in ALDH2*2 human iPSC and mouse models of diabetes and alcohol exposure and diabetes. These findings suggest that alcohol consumption should be considered with caution in ALDH2*2 carriers and that empagliflozin might potentially mitigate risk for CAD in this population. | Hongchao Guo*, Yu X, Liu Y, Paik DT, David T Paik, Justesen JM, Chandy MJ, Jahng JW, Zhang TJ, Wu WJ, Rwere F, Pokhrel S, Simon DJ, Manhas A, Zhang A, Chen CH, Rivas MA, Gross ER, Mochly-Rosen D, Wu JC. | |
Endogenous Retrovirus-Derived lncRNA BANCR Promotes Cardiomyocyte Migration in Humans and Non-human Primates | We discovered a heart-specific and endogenous retrovirus-derived long-coding RNA, BANCR, and revealed the roles of BANCR in cardiac migration and development. The human data also suggested BANCR as potential therapeutic target for pediatric dilated cardiomyopathy. | Wilson K*, Ameen M*, Hongchao Guo*, Abilez OJ, Tian L, Mumbach MR, Diecke S, Qin XL, Liu YG, Yang HX, Ma N, Gaddam N, Cunningham N, Gu MX, Neofytou E, Prado M, Hildebrandt TB, Karakikes I, Chang HY, Wu JC. | |
Single-Cell RNA Sequencing of Human Embryonic Stem Cell Differentiation Delineates Adverse Effects of Nicotine on Embryonic Development | We developed a human embryonic stem cell (hESC) model to demonstrate how nicotine—which is also found in e-cigarette liquid and smoking cessation aids—adversely affects different cell types in the developing embryo. Our single-cell RNA sequencing data suggest direct adverse effects of nicotine on hESC differentiation at the single-cell level and offer a new method for evaluating drug and environmental toxicity on human embryonic development in utero. | Hongchao Guo, Tian L, Zhang JZ, Kitani T, Paik DT, Lee WH, Wu JC. |
The Guo Lab Research Team