Heart disease is the leading cause of mortality in the US and is often preceded by pathological cardiac hypertrophy due to chronic hypertension and sustained increases in cardiac afterload. While initially an adaptive response to maintain cardiac output and systemic blood supply, pathological cardiac hypertrophy eventually leads to a decompensated state of heart failure (HF). The initial adaptive aspect of hypertrophic growth requires increases in protein synthesis, which must be balanced by adequate protein folding, and degradation of misfolded, potentially toxic proteins. Without this balance, cardiac myocytes cannot maintain protein homeostasis, or proteostasis, which threatens functional cellular integrity and viability. The Blackwood laboratory is focused on elucidating novel mechanisms whereby proteostasis machinery in the heart protects against chronic systemic diseases, such as HF and global metabolic syndrome, translating these discoveries into novel therapeutics, and evaluating their efficacy in preclinical models of HF. Specifically, we have three directives guiding our research program: (1) Elucidate novel mechanisms for heart-directed inter-organ communication during metabolic syndrome (i.e. Axes of Cardiometabolic Health); (2) Determine mechanisms contributing to atrial dysfunction and remodeling during HF; and (3) Determine the contributions of resident cardiac fibroblasts to the pathogenesis of HF.
Featured Publications
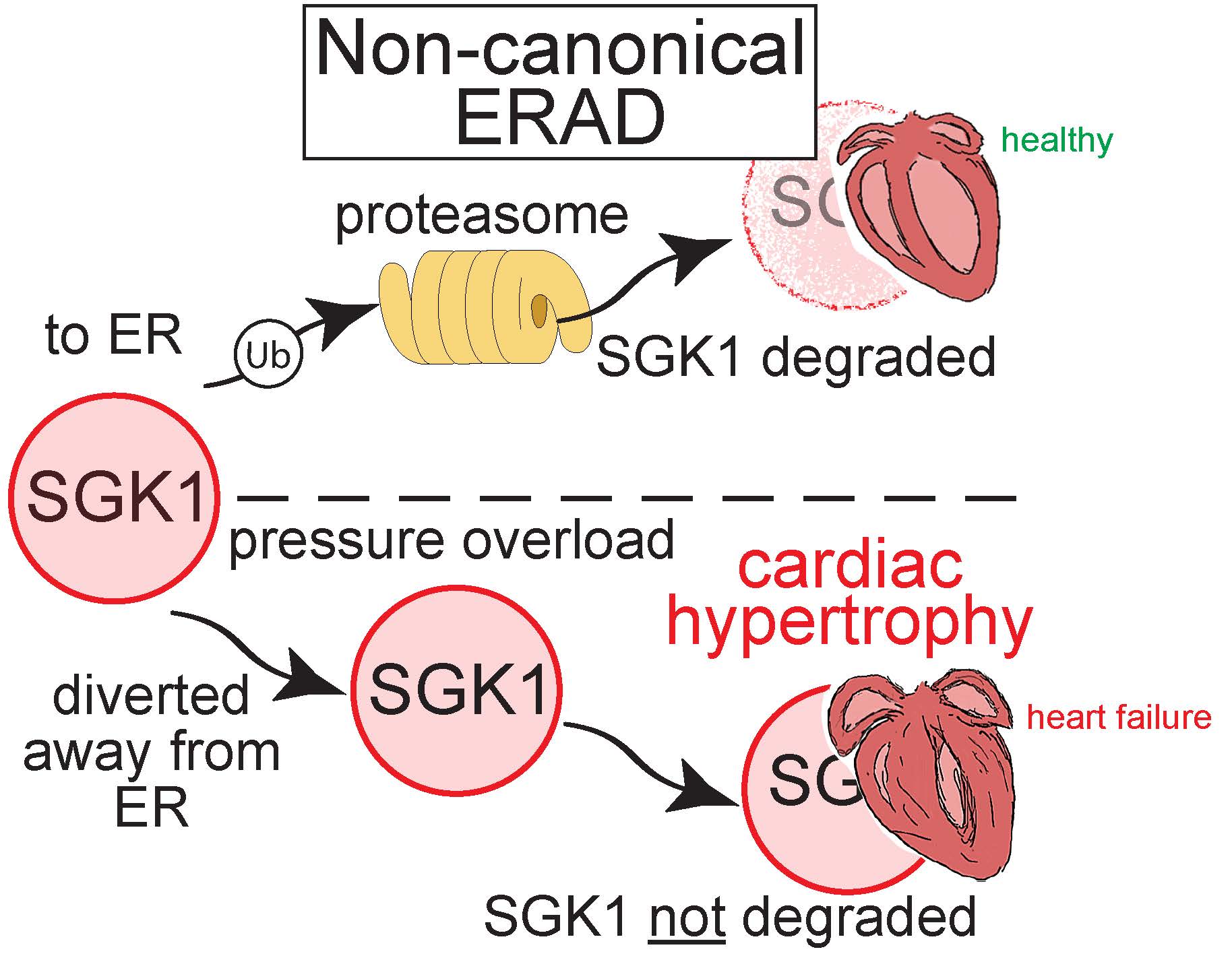
Noncanonical Form of ERAD Regulates Cardiac Hypertrophy. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.122.061557 | Breaking the dogma of how proteins are recognized for proteasome-mediated degradation at the endoplasmic reticulum (ER), this study identifies an entire new class of substrates degraded by ER-associated degradation (ERAD) and their importance in regulating cardiac myocyte function and hypertrophy in the setting of pressure overload-induced heart failure. | Blackwood, E. A., MacDonnell, L. F., Thuerauf, D. J., Bilal, A. S., Murray, V. B., Bedi, K. C., Jr, Margulies, K. B., & Glembotski, C. C. (2023). Noncanonical Form of ERAD Regulates Cardiac Hypertrophy. Circulation, 147(1), 66–82. | |
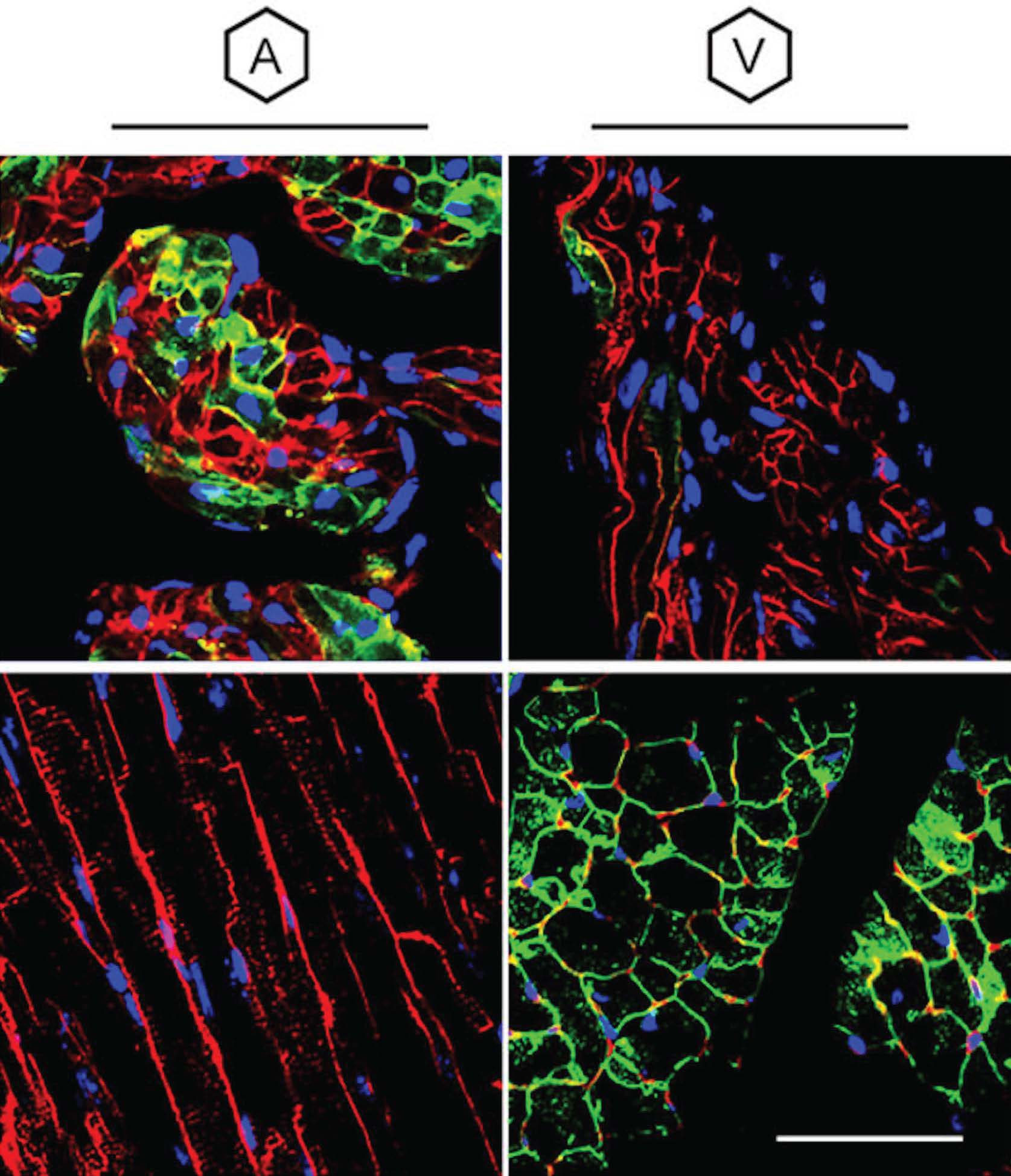
Optimizing Adeno-Associated Virus Serotype 9 for Studies of Cardiac Chamber-Specific Gene Regulation. Optimizing Adeno-Associated Virus Serotype 9 for Studies of Cardiac Chamber-Specific Gene Regulation. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.120.052437 | Despite cardiac myocytes in the atrial and ventricular chambers of the heart displaying tremendous differences in biology and function, current AAV-based strategies for gene targeting have only allowed for ubiquitous targeting of both cell types presenting major gaps in research potential and therapeutic development. In this landmark study, novel technologies were developed to allow for selective targeting of either atrial or ventricular myocytes with high fidelity using AAV viral vectors. | Bilal, A. S., Blackwood, E. A., Thuerauf, D. J., & Glembotski, C. C. (2021). Optimizing Adeno-Associated Virus Serotype 9 for Studies of Cardiac Chamber-Specific Gene Regulation. Circulation, 143(20), 2025–2027. | |
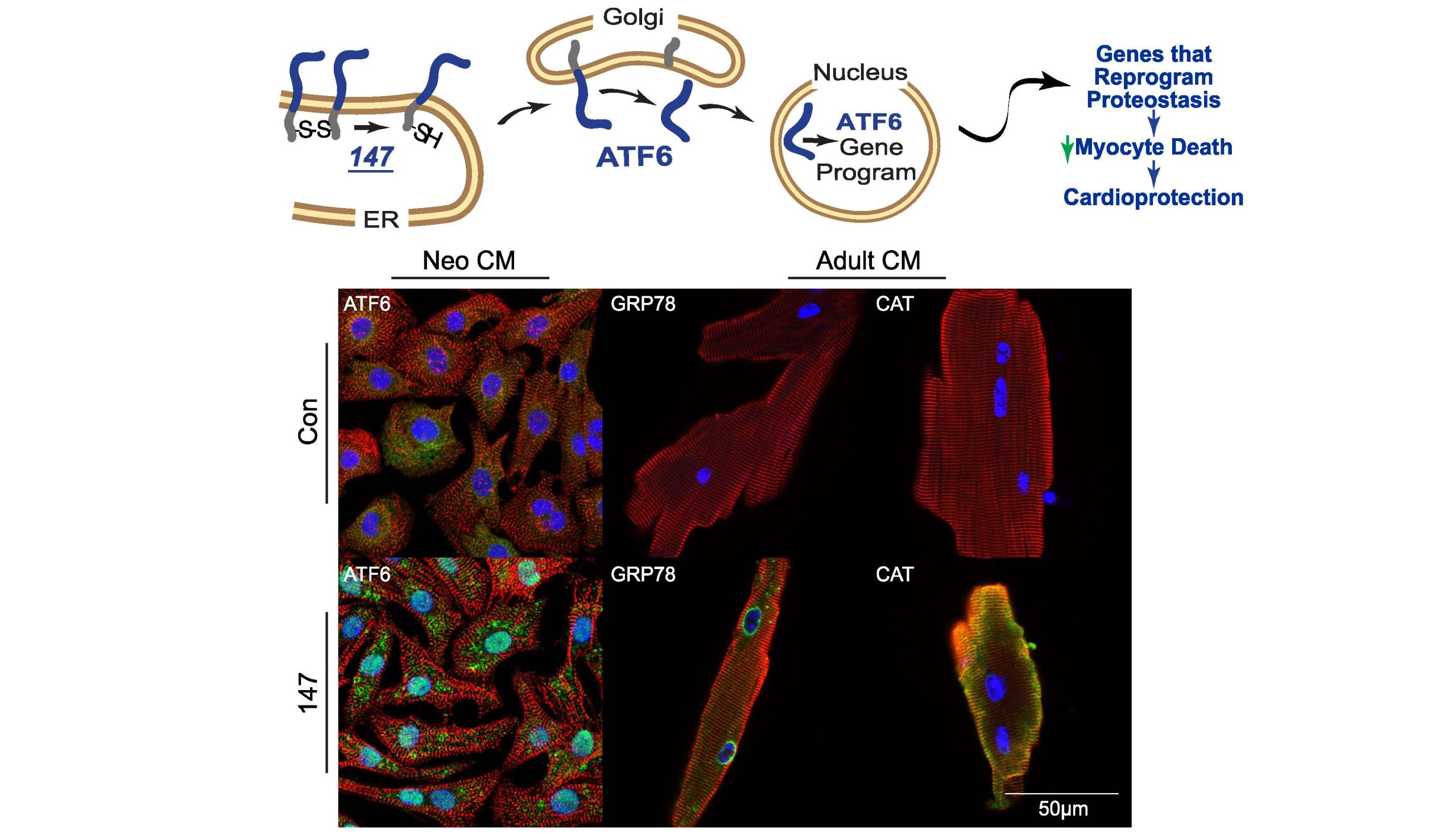
Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. https://www.nature.com/articles/s41467-018-08129-2 | The unfolded protein response (UPR) has long been sought after as a potential therapeutic target for numerous diseases including cancer, Alzheimer’s disease, diabetes, and cardiovascular disease. Despite its enormous potential as a target to treat these diseases, it’s previously thought to be “undruggable”. Here, first-in-class small molecule activators were identified with selective activity specifically for the ATF6-arm of the UPR with demonstrated incredible efficacy for treating a widespread variety of diseases predicated upon protein misfolding. | Blackwood, E. A., Azizi, K., Thuerauf, D. J., Paxman, R. J., Plate, L., Kelly, J. W., Wiseman, R. L., & Glembotski, C. C. (2019). Pharmacologic ATF6 activation confers global protection in widespread disease models by reprograming cellular proteostasis. Nature communications, 10(1), 187. |
The Blackwood Lab Research Team
 Erik Blackwood, PhD Assistant Professor | ||
 Abbey Mahnke, BS Research Associate |  Alina Bilal, MS Doctoral Student |  Lyndsay Campbell, BS Lab Manager |
 Sean Noudali, MS Doctoral Student |