
The Cardiac Recovery Laboratory
The Cardiac Recovery Laboratory’s research is focused on cardiac recovery associated with unloading and mechanical circulatory support (MCS) in the chronic heart failure (HF) setting and the acute setting (i.e. acute HF/cardiogenic shock). We have published original work generated both in the clinical arena and in our laboratory which led to the founding and establishment of the award-winning multidisciplinary Utah Cardiac Recovery Program (UCAR). The research initiatives of UCAR are developed in close connection and alignment to the development of its clinical aspects. This parallel development facilitates a full circle bidirectional synergy which is mutually beneficial to the research and clinical potentials.
Visit Cardiac Recovery Lab Website
Featured Publications
 | Enhancing mitochondrial pyruvate metabolism ameliorates ischemic reperfusion injury in the hearthttps://insight.jci.org/articles/view/180906 | We investigated a pharmacological approach of MCT4 inhibition to boost pyruvate metabolism and reduce the damage after a heart attack, which is a leading cause of death worldwide and a primary cause of heart failure. | Visker JR, Cluntun AC, Velasco-Silva JN, Eberhardt DR, Cedeno-Rosario L, Shankar TS, Hamouche R, Ling J, Kwak H, Hillas JY, Aist I, Tseliou E, Navankasattusas S, Chaudhuri D, Ducker GS, Drakos SG, Rutter J |
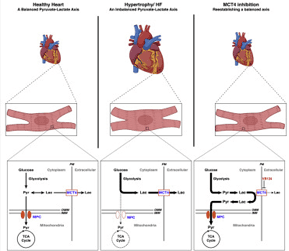 | The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. https://www.sciencedirect.com/science/article/pii/S1550413120306586 | Myocardial MPC expression coincides with LVAD-mediated recovery in chronic HF patients. Loss of the MPC in cultured cells and in murine hearts is sufficient to induce hypertrophy and HF. MPC overexpression attenuates drug-induced hypertrophy in a cell-autonomous manner. Inhibition of MCT4 can mitigate hypertrophy in cultured cardiomyocyte and in mice. | Cluntun AC*, Badolia R* (co-first authors), Lettlova S, Parnell KM, Shankar T, Diakos NA, Olson KA, Taleb I, Tatum SM, Berg JA, Cunningham CN, Van Ry T, Bott AJ, Thodou A, Fogarty S, Skedros S, Swiatek WI, Yu X, Luo B, Merx S, Navankasattusas S, Cox JE, Ducker GS, Holland WL, McKellar SH, Rutter J, Drakos SG. The Pyruvate-Lactate Axis Modulates Cardiac Hypertrophy and Heart Failure. Cell Metabolism, In Press |
 | Cardiac-specific deletion of voltage dependent anion channel 2 leads to dilated cardiomyopathy by altering calcium homeostasishttps://www.nature.com/articles/s41467-021-24869-0 | our findings demonstrate that VDAC2 plays a crucial role in cardiac function by influencing cellular calcium signaling. Through this unique role in cellular calcium dynamics and excitation-contraction coupling VDAC2 emerges as a plausible therapeutic target for heart failure. | Shankar TS, Ramadurai DKA, Steinhorst K, Sommakia S, Badolia R, Krokidi AT, Calder D, Sander P, Kwon OS, Ling J, Dendorfer A, Xie C, Kwon O, Cheng EHY, Whitehead KJ, Richardson RS, Sachse FB, Schredelseker J, Spitzer KW, Chaudhuri D, Drakos SG. Cardiac-specific deletion of voltage dependent anion channel 2 leads to dilated cardiomyopathy by altering calcium homeostasis. Nature Communications, In Press |
The Cardiac Recovery Lab Team

Stavros Drakos, MD, PhD
Professor

Jing Ling
Sr. Lab Specialist/Lab Manager

Sutip Navankasattusas, PhD
Sr. Research Associate

Joe Visker, PhD
Postdoctoral Fellow

Christos P. Kyriakopoulos,MD
Postdoctoral Fellow

Anu Shankar, PhD
Postdoctoral Research Associate

Eleni Tseliou,MD,PhD
Physician Scientist Training Track

Dallen Calder
Undergraduate Student

Sophia Skedros
Undergraduate student

Iosif Taleb, MD
Post Doctoral Fellow

Elizabeth Lynn Stauder
Medical Student

Kimiya Nourian
Medical Student

