
July 2022 Newsletter
Nora Eccles Harrison Cardiovascular Research and Training Institute (CVRTI)
Annual Newsletter | July 2022
Welcome to our annual Nora Eccles Harrison Cardiovascular Research and Training Institute (CVRTI) Newsletter!
Established in 1967, the CVRTI is a freestanding 30,000 ft2 cardiac research institute on The University of Utah Health Sciences campus that provides a highly integrated approach to the study of basic and translational cardiac biology. CVRTI personnel include 13 full-time faculty Investigators (PhDs, MDs, MD/PhDs, and DVM/PhDs), with over 100 trainees (postdocs, graduate students, undergraduates) and dedicated research staff. Our faculty Investigators are drawn from three Colleges and six Departments across the University of Utah. In this newsletter, we take you through a journey of featured highlights of our amazing faculty, their productivity, construction of our New Wing addition, and Institute wide activities of the past year.
Robin Shaw, MD, PhD
Director, CVRTI
CVRTI: Basic Advances and Bench to Bedside Solutions
Over its 55 years, the CVRTI and cardiovascular research at The University of Utah have generated a storied history. From the Jarvik Heart (first implanted total artificial heart), to the identification of genetic and acquired Long QT syndrome, to the experiment and theory behind cardiac excitation which laid the theoretical groundwork for sophisticated cardiac electrical mapping and ablation today, the CVRTI has been on the forefront of basic discoveries which then make their way to patient care. These discoveries have had a powerful impact on advancing the treatment of cardiovascular disorders and improving the health of cardiac patients worldwide.
More recently, Dr. Stavros Drakos and his colleagues have advanced the concept that failing heart muscle is sufficiently dynamic to be recovered. Dr. Drakos, highlighted below, focuses on both understanding which cohort of patients with advanced heart failure have the potential to recover without a heart transplant and discovering the molecular recovery pathways so that much needed treatments can be developed.
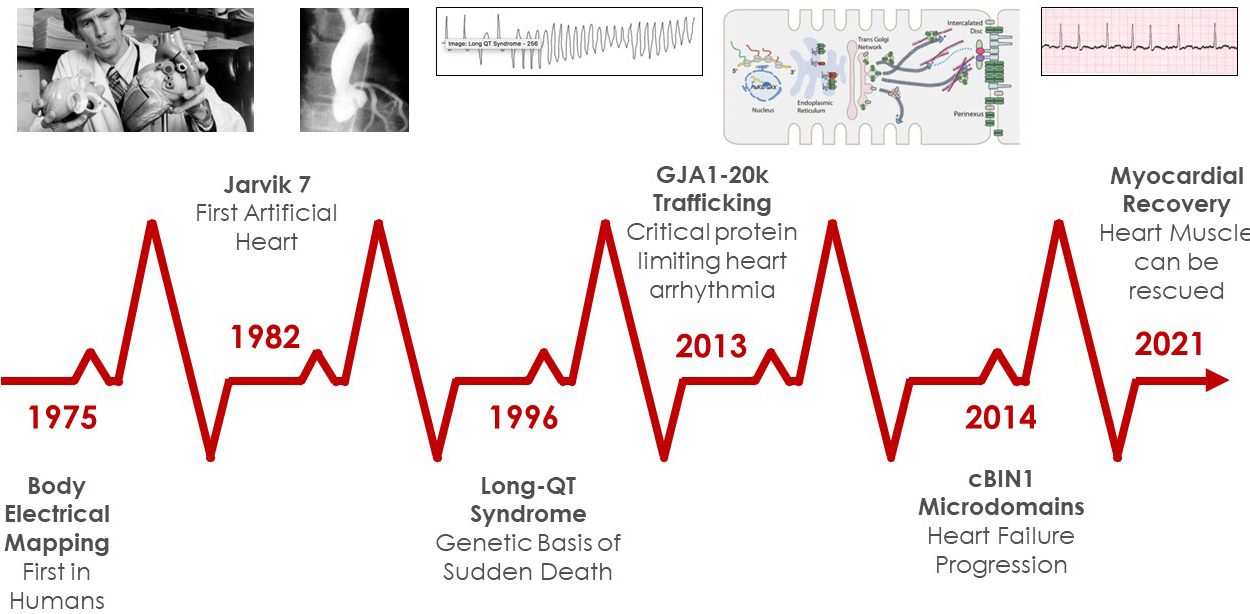
Two programs are also underway that address major current unmet needs in cardiovascular medicine. CVRTI Investigators identified a protein, called GJA1-20k, that is an essential trafficking subunit for the formation of communication gap junction channels which allow heart cells to share electrical signals with each other. Patients with severe arrhythmia syndromes, such as genetic Arrhythmogenic Cardiomyopathy, have decreased amounts of GJA1-20k. CVRTI Scientists are teaming up with the biotechnology company Renovacor to develop GJA1-20k into a therapy for patients with several genetic subtypes of Arrhythmogenic Cardiomyopathy.
At the same time, CVRTI Investigators have taken on the challenge of addressing much needed muscle specific therapy for chronic heart failure. CVRTI Investigators have also discovered a protein called cardiac BIN1 (cBIN1), which is a membrane architectural protein that organizes the calcium handling machinery inside heart cells. Such machinery is responsible for our hearts’ ability to both contract and relax and is down regulated in most forms of chronic heart failure. Mouse studies have identified that replacing cBIN1 will improve heart contraction and relaxation, can rescue heart failure, and limit heart failure associated mortality. Our Investigators are now using preclinical models to explore how the protein can be administered safely in humans and are organizing the effort that should lead a clinical trial in the next five years.
Thank you for taking the time to explore the CVRTI. We are committed to understanding the biology of some of the most intractable cardiovascular problems, and to develop solutions for them.
New Wing Addition is Under Construction!

The CVRTI is expanding with the addition of a 10,000 sq. ft. new wing generously funded by the Nora Eccles Treadwell Foundation, University of Utah’s Senior Vice President of Health Sciences, Dr. Michael Good, and University of Utah’s President Taylor Randall. Construction began in May 2022 with an expected completion date of Spring 2023. The addition builds upon the strong growth of wet-bench research at the CVRTI and the University as a whole.
The design will consist of a new two-story building including wet lab space with additional PI and trainee offices, two kitchens, and a gathering space overlooking the Wasatch Mountains. The New Wing will also include a mothers’ room on the second floor and a 1,300 square foot freezer farm in the basement.
Follow us on Twitter: @NEH_CVRTI
CVRTI Seminar Series
This year we benefited from many amazing speakers for our 2021-2022 seminar series!
Our Distinguished External Speakers the past season were
- Neil C. Chi, MD, PhD
- Yoram Rudy, PhD, FAHA, FHRS
- Mario Delmar, MD, PhD
- J. Kevin Donahue, MD
- Kirk U. Knowlton, MD, FACC, FAHA
- Jose Jalife, MD, PhD
- Jonathan Kirk, PhD

Review the presentations on our YouTube channel or on our website!
Be sure to join us when the seminar series returns in September, on Thursday’s 12-1 pm MT!
Utah Cardiac Recovery Symposium (U-CARS)
The 2022 Utah Cardiac Recovery Symposium (U-CARS), organized by Drs. Drakos, Kfoury, Selzman, and Stehlik, brought together world renowned speakers on myocardial recovery, stimulating in-depth discussions and highlighting groundbreaking research from around the world.
This year’s symposium was held virtually and attracted over 900 attendees.
U-CARS Website
Featured CVRTI Investigator

Stavros Drakos, MD, PhD
Dr. Stavros George Drakos is an investigator at the Nora Eccles Harrison Cardiovascular Research and Training Institute (CVRTI) and a Professor of Medicine Cardiology at The University of Utah Health and School of Medicine. He serves as the Medical Director of the institution’s historic Cardiac Mechanical Support/Artificial Heart Program, Co-Chief Heart Failure & Transplant and Director of Research for the Division of Cardiology. Dr. Drakos and his team published bench to bedside studies which led to the establishment of the award-winning Utah Cardiac Recovery Program (UCAR). He is the chairperson of the Clinical Integrative Cardiovascular and Hematological Sciences (CCHS) at the NIH and a visiting professor in academic medical institutions nationally and internationally. He has been co-chairing the annual international Utah Cardiac Recovery Symposium (UCARS) and the NIH Working Group on Myocardial Recovery.
Over the years, Dr. Drakos mentored several individuals who are now independent faculty in academic medical centers in the United States and overseas. As a testament to his excellence across the breadth of academic medicine and research, he was inducted into the prestigious ASCI (American Society for Clinical Investigation) and was recently selected by The University of Utah for the prestigious H.A. and Edna Benning Presidential Endowed Chair.
Representative Recent Publications
Over the past year, all of our investigators have published well. Some high impact work in cardiac metabolism and mitochondrial biology (Chaudhuri and Shaw), myocardial recovery (Drakos), and genetics (Tristani-Firouzi) are featured below.
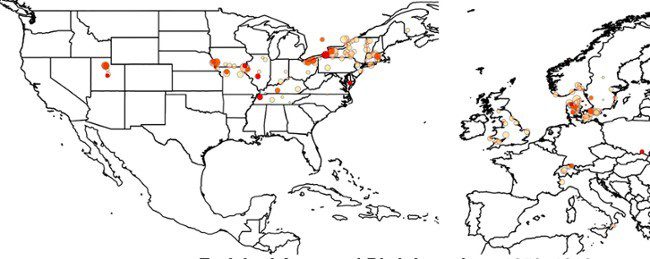
The history and geographic distribution of a KCNQ1 atrial fibrillation risk allele
Hateley et. al., Nat. Commun., 2021, Tristani-Firouzi Lab
This first-of-its-kind study highlights the power of merging human genetics and human history in order to understand atrial fibrillation in a historical context. The collaborative effort between the University of Utah, Intermountain Cardiology, and AncestryDNA broadens our understanding of atrial fibrillation to include the history of our ancestral origins and population movement across time and continents.

Protective mitochondrial fission induced by stress-responsive protein GJA1-20k
Shimura et. al, eLife, 2021, Shaw Lab
After 30 years of research in ischemic-preconditioning, researchers have not been able to identify the central regulator. The Shaw Lab has now identified the long sought regulator of ischemic preconditioning which is a molecule we named GJA1-20k. They have found that this stress responsive molecule works with the actin cytoskeleton to split mitochondria in a protective fashion, protecting cells and larger organs against ischemic stress. This achievement also provides a novel therapeutic option to protect organs undergoing anticipated ischemia and stress-related injury.
Cardiac-specific deletion of voltage dependent anion channel 2 leads to dilated cardiomyopathy by altering calcium homeostasis
Shankar et. al., Nat. Commun., 2021, Drakos Lab
VDAC2 is a calcium channel present in the mitochondria of the cells of the heart. The Drakos Lab found that removing VDAC2 in mice hearts leads to heart failure by reducing the calcium uptake which led to impaired squeezing/pumping function of the heart. Re-introducing VDAC2 in these mice, resulted in improved squeezing/pumping function and overall cardiac health. Using a VDAC2 activator, efsevin, in mice with heart failure we observed significant improvement of these failing hearts. All of these findings show that VADC2 can be a novel therapeutic target to treat heart failure.

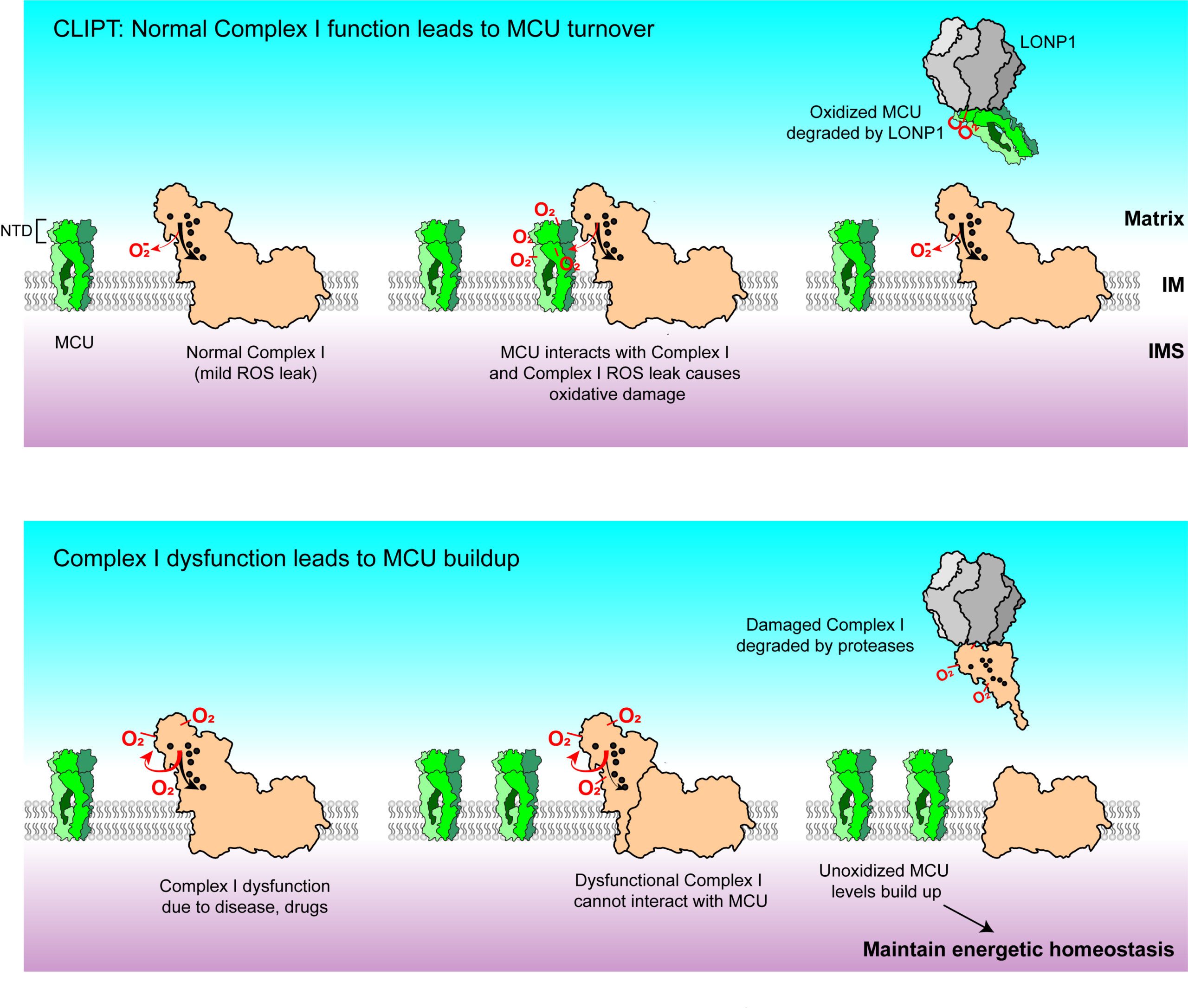
Mitochondrial calcium uniporter stabilization preserves energetic homeostasis during Complex I impairment
Balderas, et. al., Nat. Commun., 2022, Chaudhuri Lab
Inborn errors of metabolism affect about 1 in 5000 infants and are most commonly due to mutations in mitochondrial proteins. These children often suffer from heart failure, for which there are no approved therapies. In these diseases, the Chaudhuri Lab has identified a novel pathway that appears to compensate for mitochondrial dysfunction, involving a mitochondrial calcium channel. Because boosting this channel appears to prolong survival and improve function in animal models of these diseases, it may represent a novel therapeutic target.
See All CVRTI’s Publications HereGrants and Awards
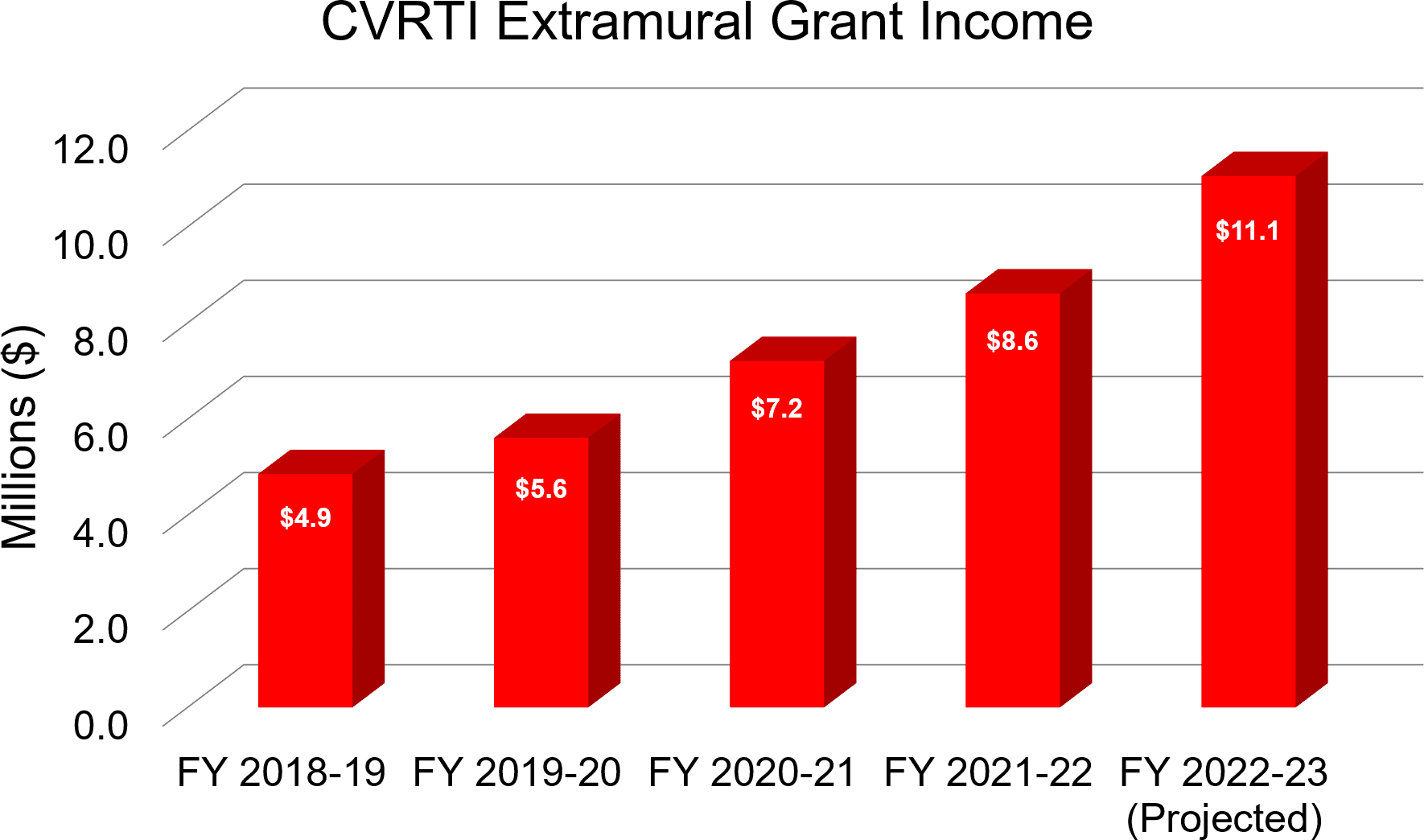
American Heart Association (AHA) Trainee Award Recipients

Characterization of Smyd1 Enzymatic Activity
Kathryn Davis, PhD Candidate
AHA Predoctoral Fellowship

A Novel Mechanism of Mitochondrial Protein Turnover in Complex I Deficient Mitochondrial Cardiomyopathy
Sandra Lee, MD/PhD Student
AHA Predoctoral Fellowship

Targeting EF Hand Domain Containing 1 Protein (EFHD1) in Cardiac Disease
David Eberhardt, PhD
AHA Postdoctoral Fellowship
National Institutes of Health (NIH) Trainee Award Recipients

Regulation of Purine Metabolism by Protein Methyltransferase Smyd1
Magnus Creed, PhD Candidate
Ruth L. Kirschstein NIH NRSA F31 Predoctoral Fellowship

Role of Structural Remodeling in Atrial Fibrillation
Eugene Kwan, PhD Candidate
Ruth L. Kirschstein NIH NRSA F31 Predoctoral Fellowship

SMYD1’s Role in Regulating Disease-Induced Remodeling and Gene Expression in the Cardiomyocyte
Marta W. Szulik, PhD
Ruth L. Kirschstein NIH NRSA F32 Postdoctoral Fellowship
Current CVRTI Cardiovascular T32 Award Recipients

New Insights Into The Pathophysiology Of Dilated Cardiomyopathy In Inherited Phosphoglucomutase I Deficiency
Bijina Balakrishnan, MS, PhD
Evaluating the Effect of Vericiguat on Peripheral Vascular Function, Patient Health Status and Inflammation in Patients with Chronic Heart Failure with Reduced Ejection Fraction
Lina Brinker, MD

cBIN1 Gene Therapy Rescues Ischemic Chronic Heart Failure
Muhammad S. Khan, PhD

The Role of Hyaluronan Synthase 2 on Endothelial Glycocalyx with Vascular Aging
Jisok Lim, PhD

The Role of Pyruvate-Lactate Metabolic Axis and Myocardial Salvage Following Ischemic-Reperfusion Injury
Joseph R. Visker, PhD, RCEP

The Role of Hyaluronan Synthase 2 on Endothelial Glycocalyx Targeted DNA-Encoded Libraries for the Discovery of Covalent Sirtuin-6 Activators with Vascular Aging
William K. Weigel, PhD

Regulation of Mitochondrial Health by Mitochondrial Derived Compartments in Cardiomyocytes
Sara Wong, PhD
Nora Eccles Treadwell Foundation and the Eccles Family:
55 Years of CVRTI support and growing
The CVRTI was the brainchild of the late, great hematologist, Dr. Max Wintrobe, who also served as the first CVRTI Director in 1967. Dr. Wintrobe spearheaded an effort for private funding to supplement federal support and he, along with prominent Utah businessman Robert S. Carter and Willard L. Eccles, created and directed the Cardiovascular Institute Foundation with the goal of raising $50,000/yr for five years. Among the numerous contributors were several members of the Eccles family including George S. Eccles, Marriner S. Eccles, and Spencer F. Eccles as well as Nora Eccles Harrison and her husband, Richard A. Harrison. This marked the beginning of the nearly 50 years of extraordinarily generous support of the CVRTI by Nora Eccles and the Nora Eccles Treadwell Foundation (NETF), created in 1962. Contributions to CVRTI from the NETF and the Eccles family have already amounted to over $60M and the NETF has recently reaffirmed its ongoing commitment to cardiovascular research with a $40M gift.

From left to right: Dr. Kenneth Spitzer, Mr. Lawrence Harrison, Mr. Robert Graham, Dr. Craig Selzman (Chief, Cardiothoracic Surgery), Mr. Spencer F. Eccles, Dr. Kathryn Econome, Ms. Katie Eccles, Dr. Robin Shaw, Dr. James Fang (Chief, Cardiology), and Ms. Tara Hitzeman
NETF funding includes support for numerous research and infrastructure grants, earlier construction of two research wings, current construction of a third additional wing, and major equipment purchases. Annual NETF grant funding includes support for the research programs of each individual CVRTI Investigator as well as the majority of our CVRTI operational expenses. The Board of Directors of the Nora Eccles Treadwell Foundation provides active and visionary leadership for the CVRTI and is currently composed of five members: Spencer F. Eccles (Chairman, nephew of Nora Eccles), Katie A. Eccles (Vice Chair, daughter of Spencer F. Eccles), Kathryn C. Econome, MD, Lawrence M. Harrison (Secretary, nephew of Richard A. Harrison), Robert M. Graham, and Kenneth W. Spitzer, PhD (former Director of CVRTI).
Nora Eccles recognized the importance of the CVRTI as a basic science institute, prioritizing fundamental and cutting-edge research. Our success is a testament to the dedication and vision of Nora Eccles and her foundation. There is a direct line from the NETF’s commitment to cardiovascular research at the CVRTI and the University of Utah, and improved knowledge and cardiac care that benefits patients in the United States and the world over.
To support the CVRTI, please contact Tara Hitzeman (Tara.Hitzeman@hsc.utah.edu).
Honors and Recognitions

H.A and Edna Benning Presidential Endowed Chair
Congratulations to Dr. Martin Tristani-Firouzi for the honor of being named as a prestigious H.A. and Edna Benning Presidential Endowed Chair.
ISHR Outstanding Investigator
Dr. Sarah Franklin was recognized with the ISHR Outstanding Investigator Award for making major and independent contributions to the advancement of cardiovascular science and leading a growing research program likely to play a major role in the future.


The American Society for Clinical Investigation
Dr. Stavros Drakos was officially inducted into the American Society for Clinical Investigation (ASCI). He was also named as a prestigious H.A. and Edna Benning Presidential Endowed Chair.
Meet the CVRTI Faculty

Ademuyiwa Aromolaran, PhD

Dipayan Chaudhuri, MD, PhD

Derek Dosdall, PhD

Stavros Drakos, MD, PhD

Sarah Franklin, PhD

Guillaume Hoareau, DVM, PhD

TingTing Hong, MD, PhD

Rob MacLeod, PhD

Joseph Palatinus, MD, PhD

Ravi Ranjan, MD, PhD

Craig Selzman, MD
Robin Shaw, MD, PhD

Martin Tristani-Firouzi, MD
Meet the Leadership

Robin Shaw, MD, PhD
Director
CVRTI Senior Advisory Committee

Willard Dere, MD

Priscilla Hsue, MD

Rob MacLeod, PhD

Wes Sundquist, PhD

Joseph C Wu, MD, PhD
Associate Director and Operations

Derek Dosdall, PhD
Associate Director

Tara Hitzeman, MPH
Operations Director
Meet the Staff

Our CVRTI Core Staff contributes substantially to our productivity and uniquely attractive research environment. It is made up of seven outstanding individuals, which includes a facilities manager who supports the research laboratories and coordinates larger projects with The University of Utah’s facilities team; a laboratory specialist, dedicated to the management of CVRTI’s operating room, and an IT specialist. Additionally, we have four individuals responsible for administration including pre-award and post-award grants management, accounting, personnel matters, and coordination of seminar series and events.
Learn More About CVRTI Core StaffCVRTI Events



From left to right: Annual Summer BBQ, Holiday party at TopGolf, and a group picture of CVRTI.
CVRTI is Hiring!

CVRTI is recruiting at all levels, including faculty for our new wing! Click on the Careers Button below for specific opportunities and feel free to email CVRTI’s Operations Director, Tara Hitzeman (Tara.Hitzeman@hsc.utah.edu), with any questions.
Careers
