
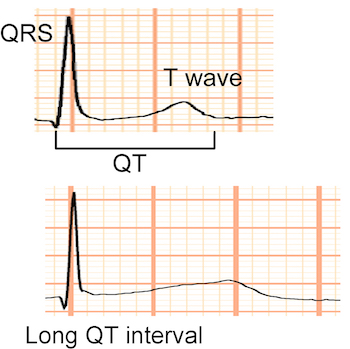
The Tristani-Firouzi Lab played an integral role in the initial characterization of the genetic and molecular basis for arrhythmia susceptibility in various forms of Long QT Syndrome (LQTS). LQTS is a genetic disorder of cardiac ion channels that causes a delay in repolarization of the ventricles, resulting in prolongation of the QT interval on the surface ECG. The delayed ventricular repolarization predisposes affected individuals to life-threatening ventricular arrhythmias. The Tristani-Firouzi Lab defined the molecular mechanisms of ion channel dysfunction in LQT1, LQT2, LQT5, and LQT7. We were the first group to design an effective trial of gene-specific therapy for the treatment of LQTS. Some of our research publications serve as landmark studies in the field of LQTS research.
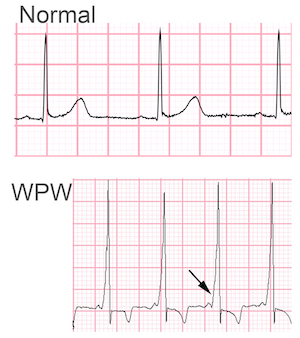
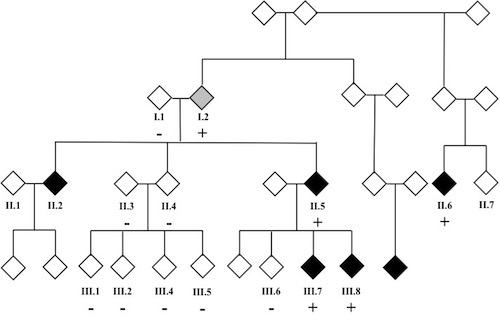
Wolff-Parkinson-White (WPW) syndrome is characterized by ventricular pre-excitation (a short PR interval and bundle branch block pattern on the surface ECG) and paroxysmal supraventricular tachycardia. Ventricular pre-excitation is due to an accessory pathway that allows for conduction between the atria and ventricles. WPW is one of the most frequent forms of supraventricular tachycardia. We recently reported the first whole exome sequencing analysis of a family with WPW. Using variant prioritization strategies designed by the Yandell lab (VAAST/pedigree VAAST coupled with the ontology-based algorithm Phevor) we generated a limited number of potential candidate alleles. Of the highly ranked alleles, only one segregated with the phenotype; a variant in the myosin heavy chain gene MYH6 c.5653G>A; p.Glu1885Lys. This variant affects a highly conserved glutamic acid in the myosin tail, is predicted to be pathogenic/deleterious by 4 separate in silico algorithms and is ranked as the strongest candidate variant by Phevor. These data support the conclusion that the MYH6 genetic variant is most likely responsible for the WPW phenotype in this family and expands the cardiac phenotypes associated with mutations in MYH6.

The Inherited Arrhythmia Clinic, founded and directed by Dr. Susan P. Etheridge and located at Primary Children’s Hospital, serves the adult and pediatric population with inherited arrhythmia syndromes. The Center provides comprehensive, state-of-the-art evaluation, diagnosis, treatment, and long-term follow-up care for patients with inherited arrhythmia syndromes in a multi-disciplinary clinic consisting of pediatric and adult electrophysiologists, a cardiovascular geneticist, genetic counselor, mid-level electrophysiology providers and translational biologists. The Inherited Arrhythmia Clinic concludes with a review of cases of the day, referred cases and a journal club review of up-to-date clinical research.
A major goal of the Tristani-Firouzi Lab is to expand our understanding of the genomic basis for arrhythmia syndromes, including atrial fibrillation (AF), WPW, LQTS, and sick sinus syndrome, by leveraging the Utah Population Database (UPDB). The UPDB houses genealogical records of more than 12 million individuals that are linked to medical records and vital statistics data. Through the identification and analysis of high-risk pedigrees, UPDB-based research defined the genetic risk factors for complex traits ranging from breast, colon and skin cancers to birth defects and congenital heart disease. Our goal is to identify novel arrhythmia disease genes that we can plug into our exiting infrastructure for functional analyses (heterologous expression systems, zebrafish assays and induced pluripotent stem-cell derived cardiomyocytes). We anticipate identifying novel disease-causing genes and alleles in both coding and non-coding regulatory regions, as well as disease-associated structural variants. These findings will allow for the design of improved strategies for risk stratification, surveillance and medical intervention.