The ability to sense transmembrane voltage is an essential feature of ion channels that control cardiac excitability. For example, when a cardiac cell is depolarized, these voltage-gated ion channels open (activate) to allow the flow of ions and in some cases subsequently close (inactivate) thereby limiting the flux of ions. The Tristani-Firouzi Lab was instrumental in defining the fundamental biophysical properties of the HERG (KCNH2) cardiac potassium channel; properties that are crucial for maintaining normal electrical stability in the heart. We are one of a handful of labs in the world that is capable of measuring gating currents, the intramembrane charge displacement associated with re-arrangements of the voltage-sensing domain. Using these and other techniques, we defined the molecular steps that couple movement of the voltage sensor to channel opening and inactivation in the HERG potassium channel.
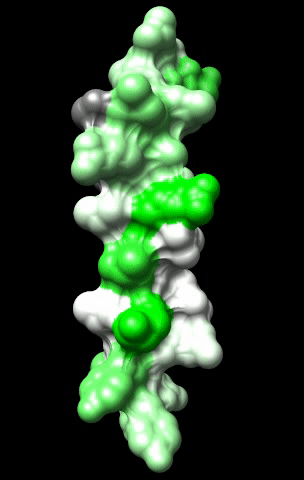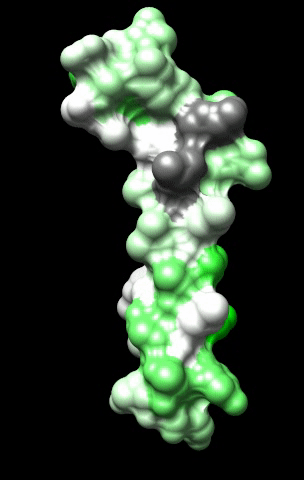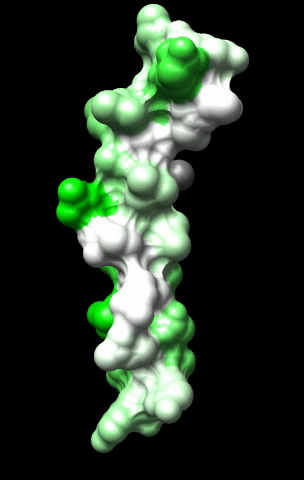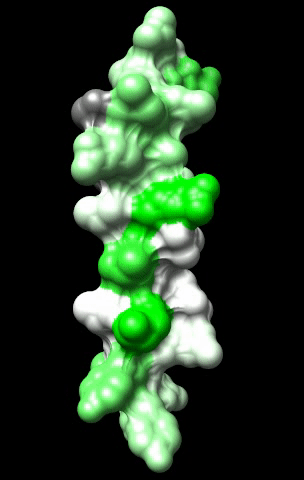
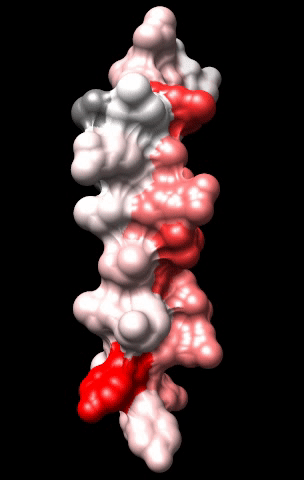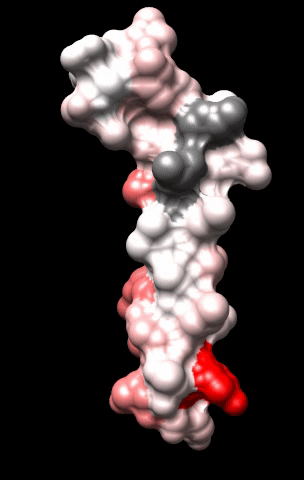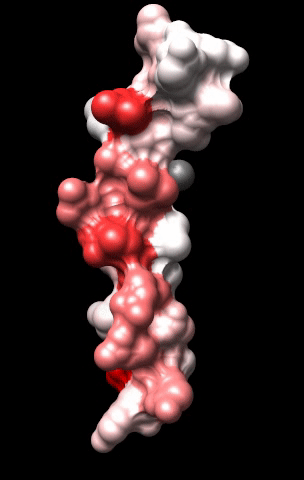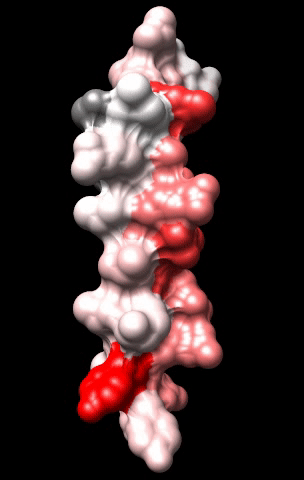
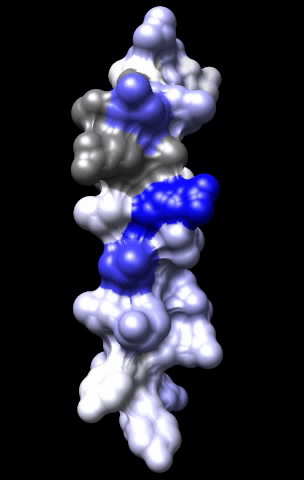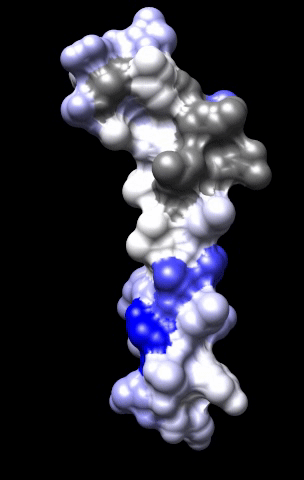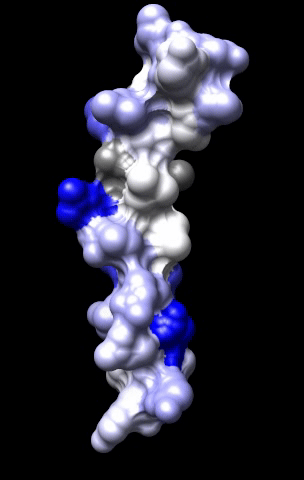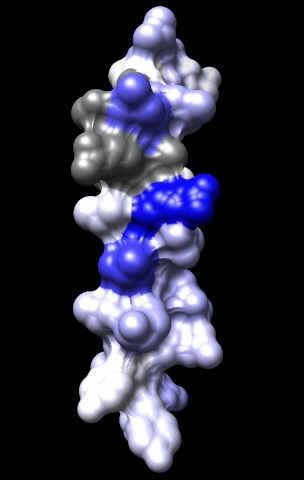
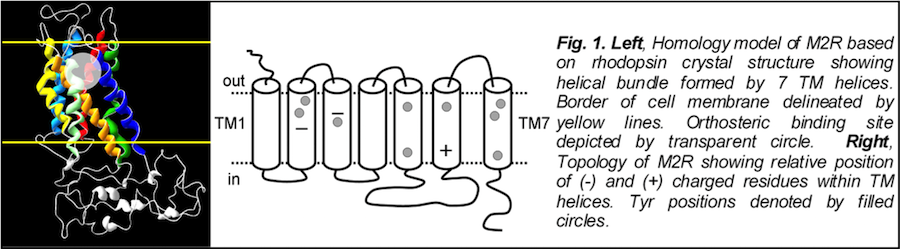
The cardiac M2R muscarinic receptor belongs to the broad class of G-protein-coupled receptors (GPCRs). While GPCRs are classically activated by external stimuli (e.g., photons, hormones, neurotransmitters, odorants and chemokines), some GPCRs are capable of sensing transmembrane voltage. Building upon our experience in measuring gating currents in voltage-gated ion channels, we demonstrated that the cardiac M2R muscarinic receptor “senses” transmembrane voltage and undergoes distinct conformational changes in response to membrane depolarization and ligand binding. This novel feature of muscarinic receptors has important implications for physiological modulation of the cardiac action potential by vagal stimulation, as well as implications for the design of pharmacological agents that target the M2R. Alterations in the voltage-sensing capacity of the M2R could disrupt the delicate balance between parasympathetic and sympathetic influences, resulting increased parasympathetic drive (susceptibility to atrial fibrillation) or decreased parasympathetic drive (syndrome of inappropriate sinus tachycardia).